Convert 22 g of carbon dioxide `(CO_(2))` into moles. (Atomic masses : `C = 12 u, O = 16 u`) - YouTube

Basic Principles and Calculations in Chemical Engineering 32e14f234b | PDF | Units Of Measurement | Mass

CO 2 results of entire MIS 11, including end of MIS 12. Dome C CO 2... | Download Scientific Diagram

Mechanically Constrained Catalytic Mn(CO)3Br Single Sites in a Two-Dimensional Covalent Organic Framework for CO2 Electroreduction in H2O | ACS Catalysis

Calculate mass of co2 produced by combustion of 8g of methane - Chemistry - Some Basic Concepts of Chemistry - 13818719 | Meritnation.com
How to calculate the work done if three moles of an ideal gas are compressed reversibly and isothermally from a volume of 10 litres to 5 litres - Quora
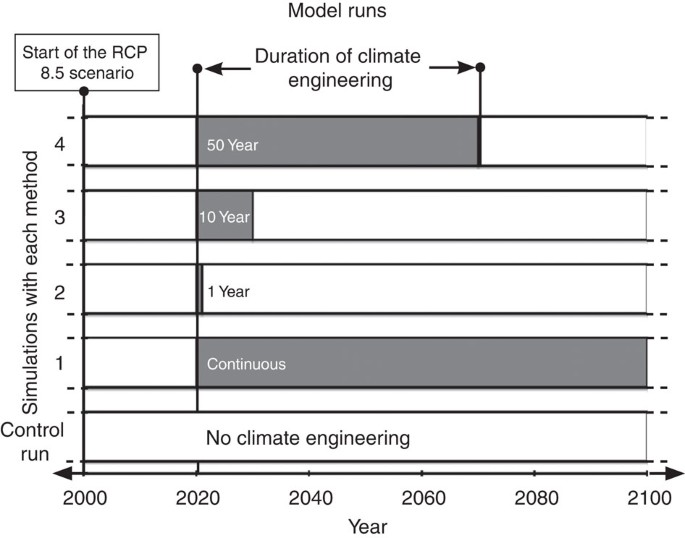
Potential climate engineering effectiveness and side effects during a high carbon dioxide-emission scenario | Nature Communications

Calculate the work done during isothermal reversible expansion of one mole ideal gas from 10 atm to 1 atm at 300 K.

Methoxy Groups Increase Reactivity of Bifunctional Tetraarylphosphonium Salt Catalysts for Carbon Dioxide Fixation: A Mechanistic Study | The Journal of Organic Chemistry

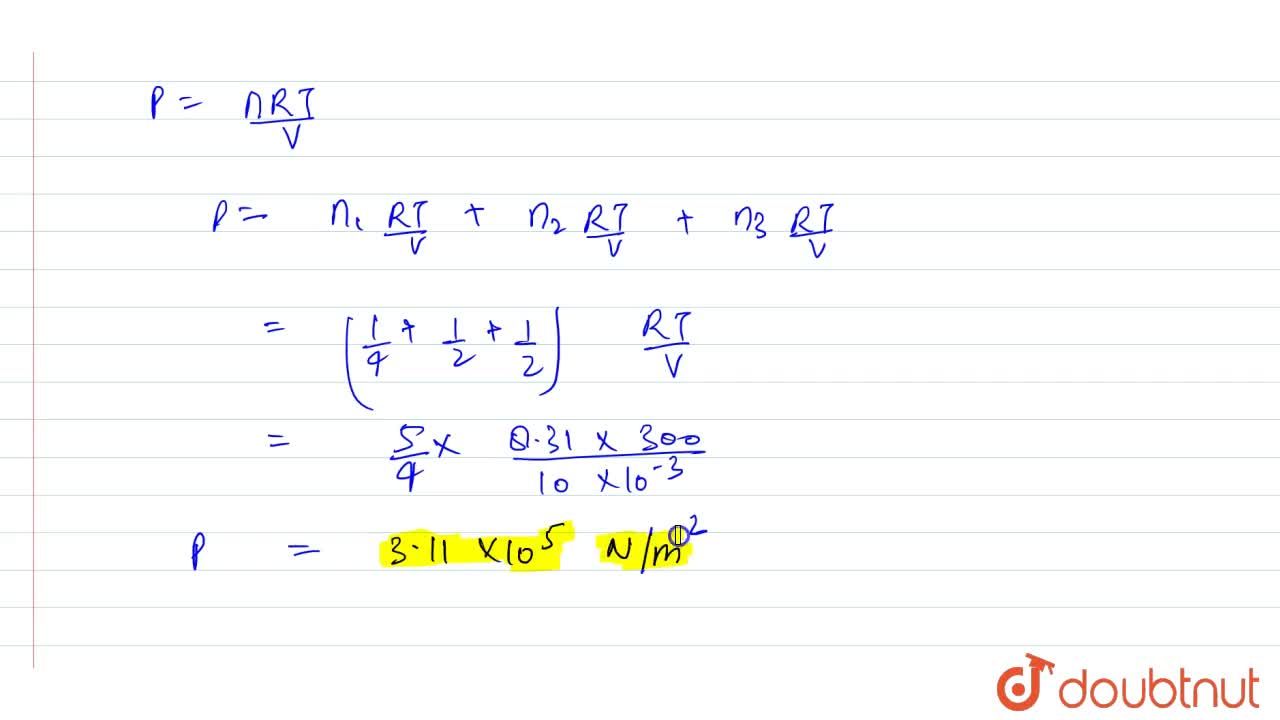
8g oxygen, 14 g nitrogen and 22 g carbon dioxide are mixed in a container of volume 4 l. Find out the pressure of the gas mixture at 27^(@)C. Given R =

The maximum work done in expanding 16g oxygen at 300K and occupying a volume of 5dm^3 isothermally until the volume become 25dm^3 is:

Metal–CO2 Electrochemistry: From CO2 Recycling to Energy Storage - Wang - 2021 - Advanced Energy Materials - Wiley Online Library

A Step toward the Quantification of Noncovalent Interactions in Large Biological Systems: The Independent Gradient Model-Extremely Localized Molecular Orbital Approach | Journal of Chemical Information and Modeling

Calculate the amount of carbon dioxide that could be produced when (i) 1 mole of carbon is burnt in air.(ii) 1 mole of carbon is burnt in 16 g of dioxygen.(iii) 2

Calculate work done when 2 mole of an ideal gas expands isothermally and reversibly at 300 K from 10 atm pressure to 2 atm pressure.