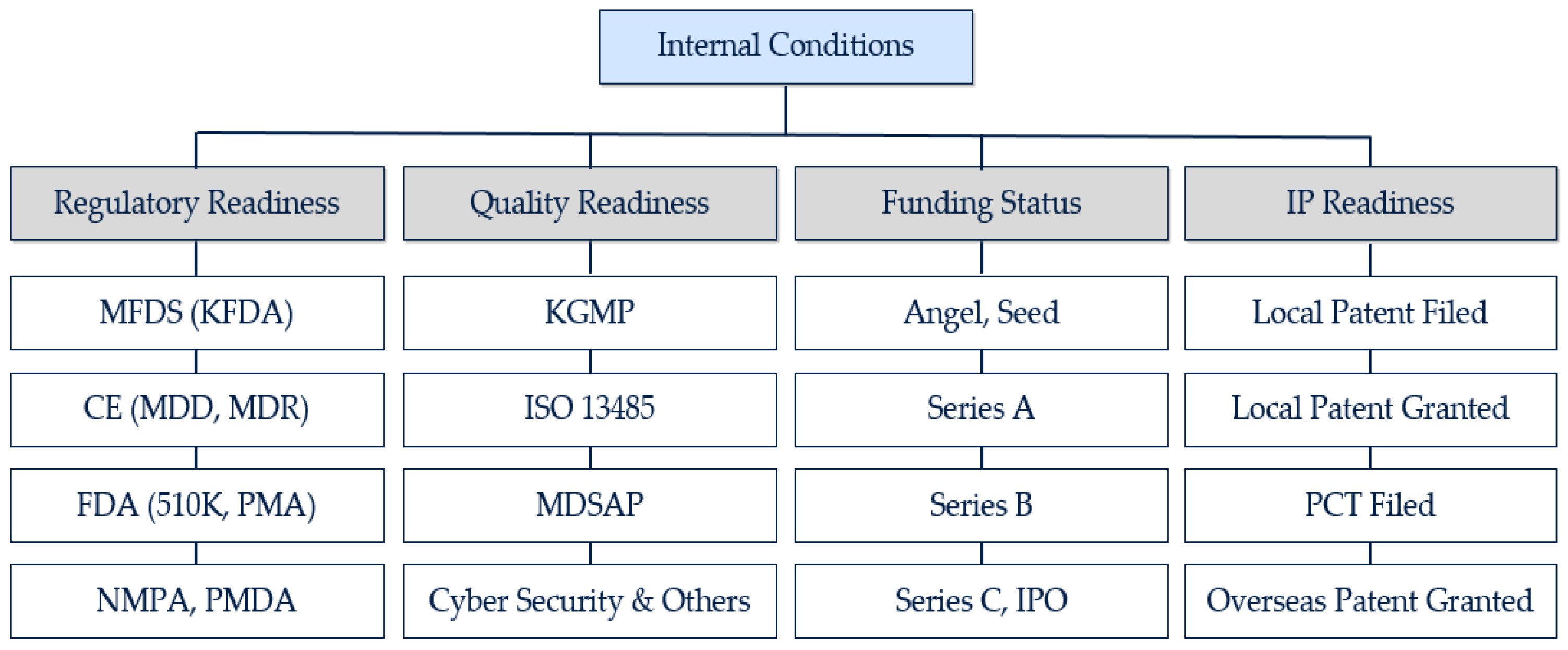
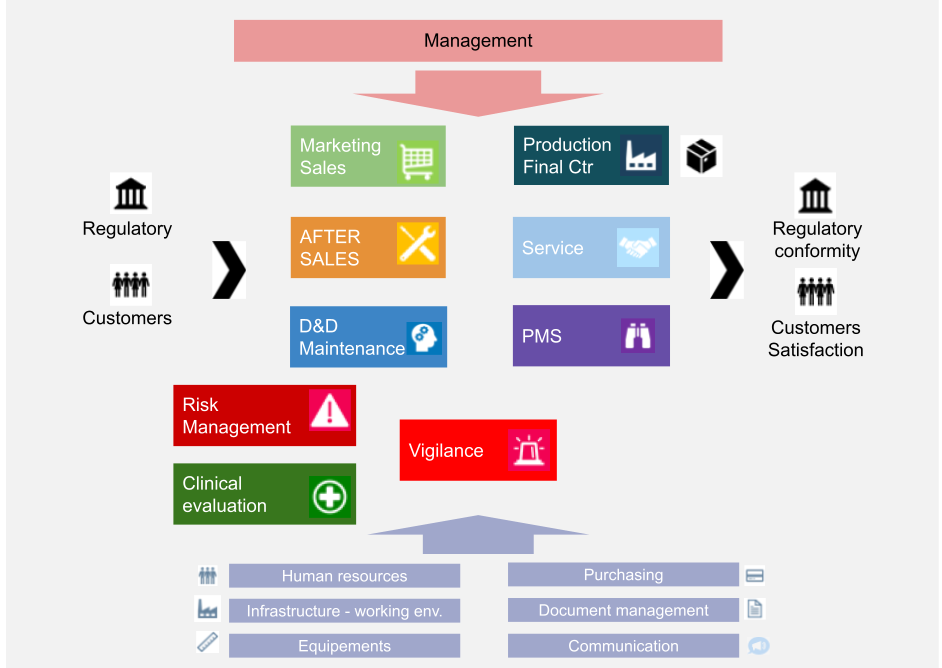
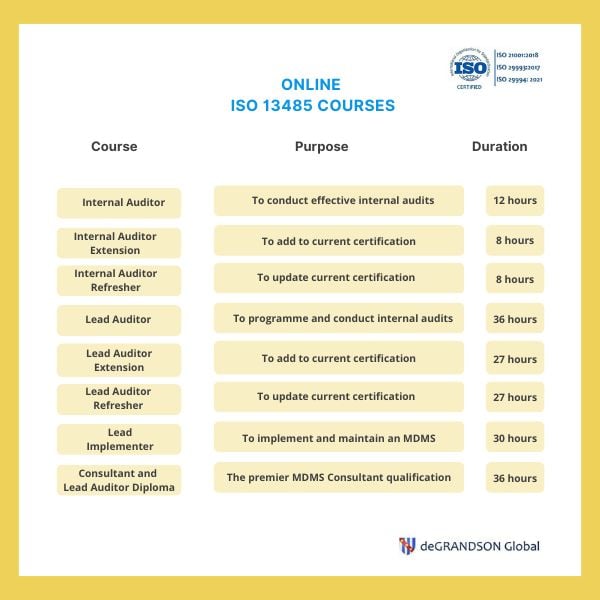
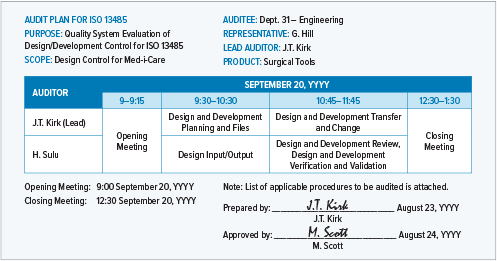
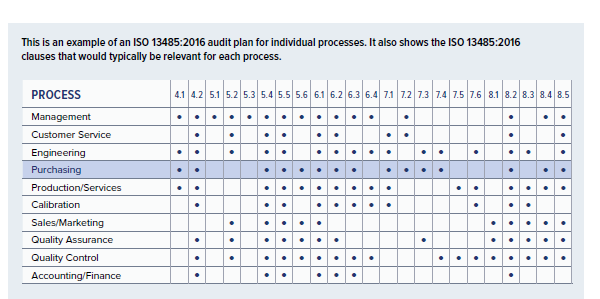
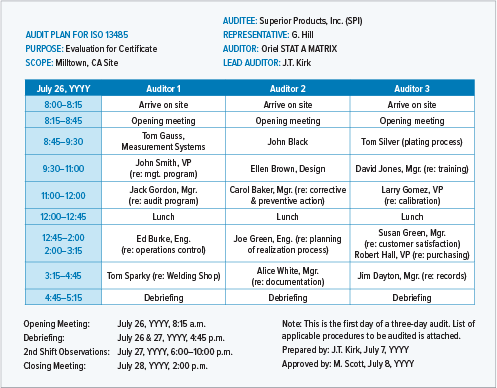
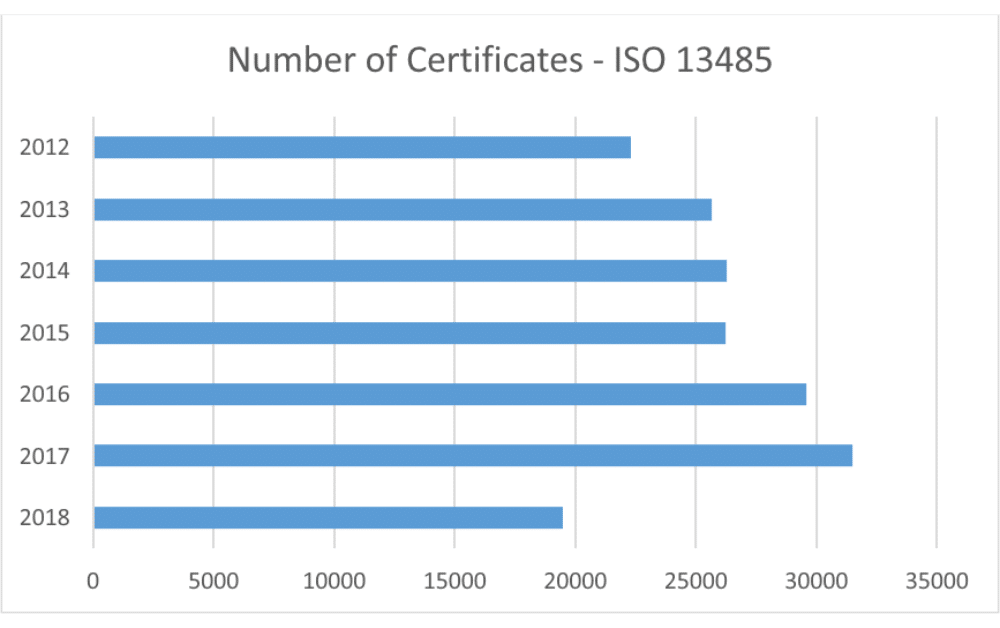
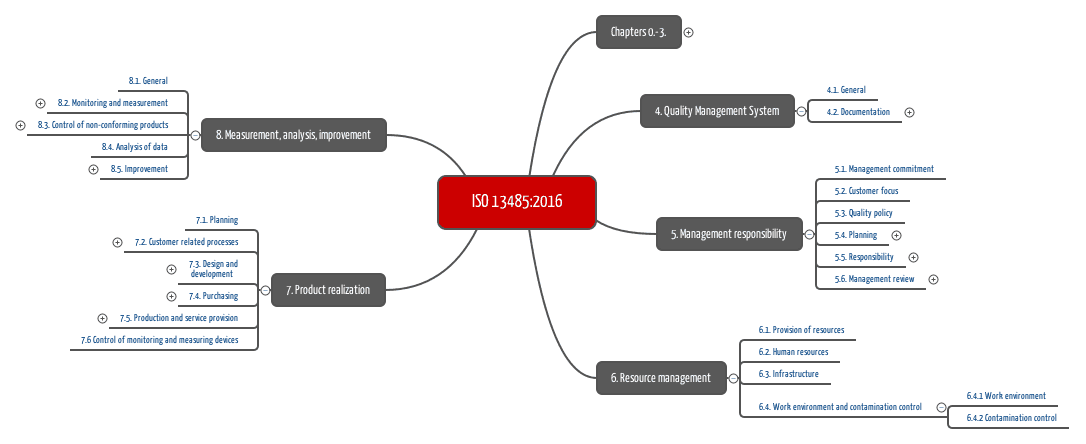
Manufacturing And Product Development Support for Medical Devices / IVDs (Class C / D) -CliniExperts

Medical Device Quality Management System Auditor (ISO 13485) - Certus Professional Certification Inc.
IAF Mandatory Document Application of ISO/IEC 17021-1 in the Field of Medical Device Quality Management Systems (ISO 13485) Issu