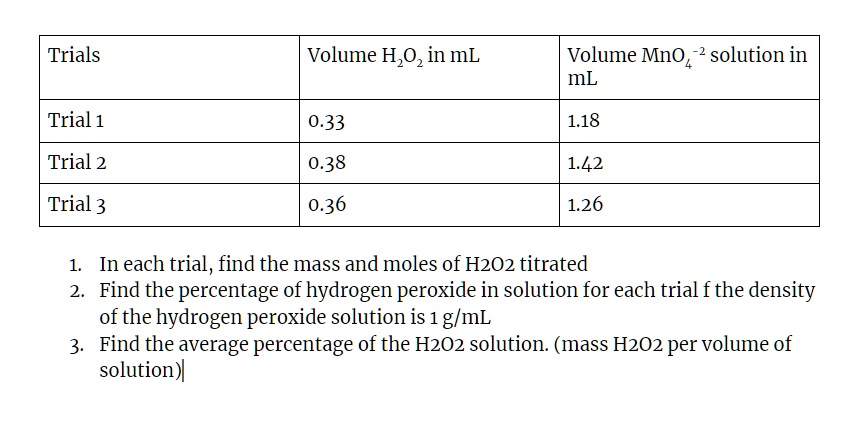
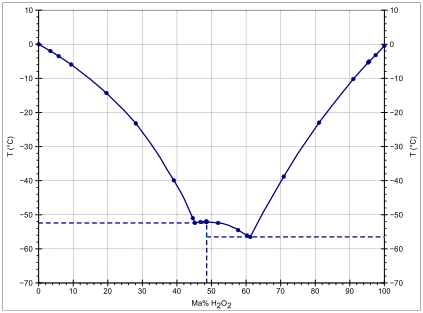
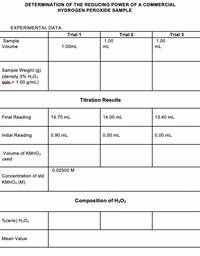
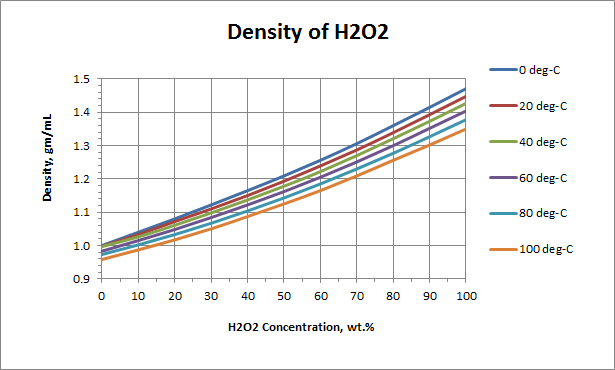
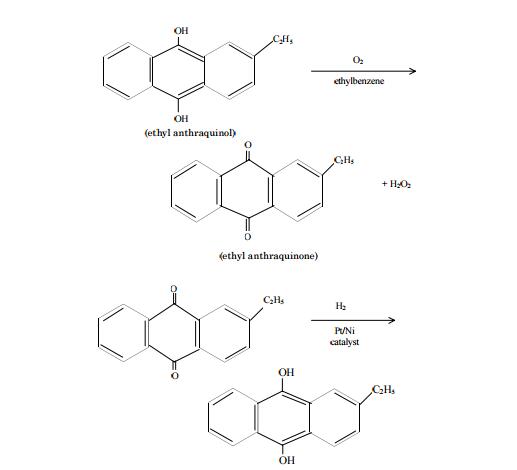
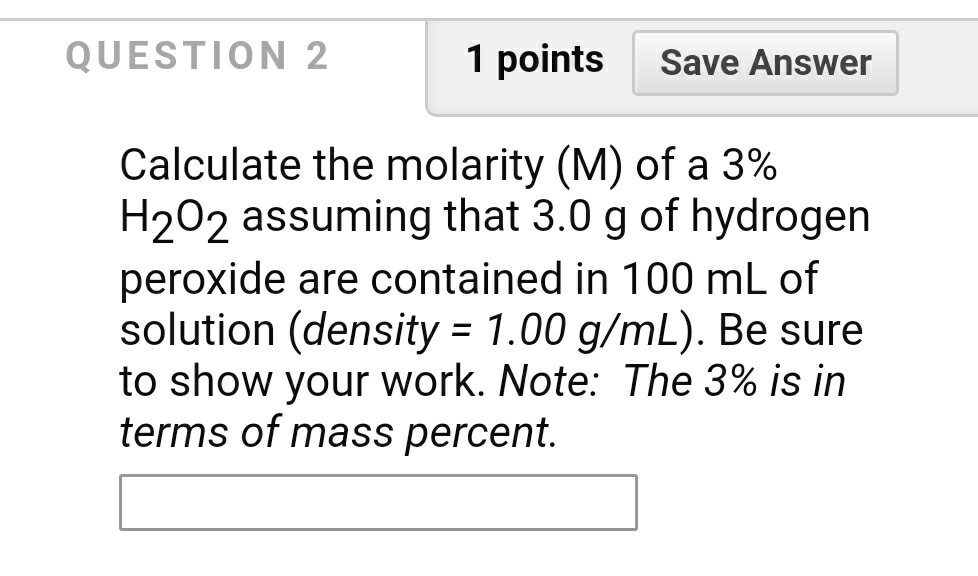
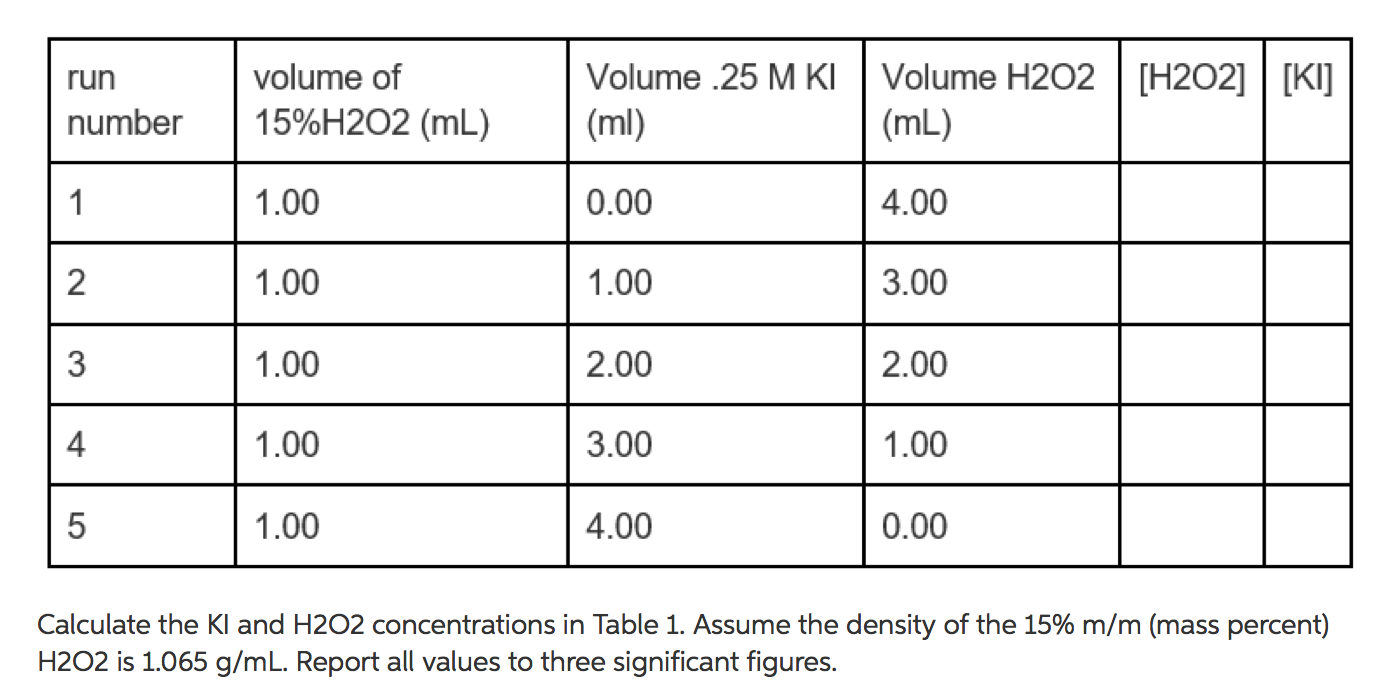
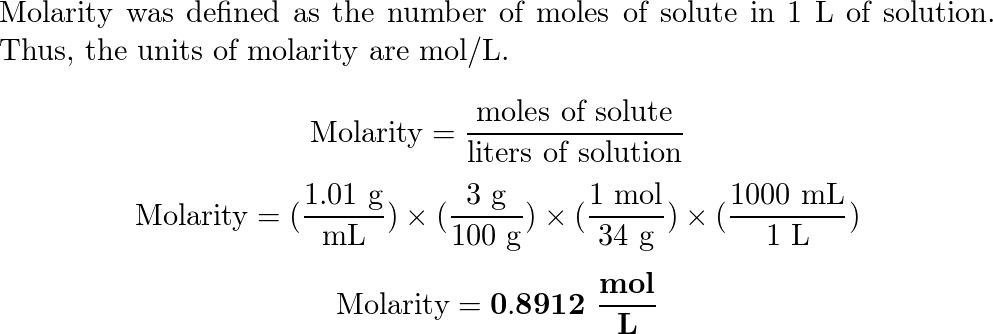OneClass: I am having a difficult time understanding this problem,can someone please assist? Than...

OneClass: If I have hydrogen peroxide, molecular weight(g/mol) is 34.02, its density (g/ml) is 1.2, i...

SOLVED: 1,100 kgIm' Whal would you expect the mass ofthat solution to be if it 309 hydrogen peroxide has density of filled container with volume of 0,03 m ? 990 kg 33kg 0000027 kg 36,666.67 kg

The strengths of 5.6 volume hydrogen peroxide (of density 1g/ mL) in terms of mass percentage and molarity (M) , respectively are : (Take molar mass of hydrogen peroxide as 34g / mol )

Table 1 from Biodecomposition of Hydrogen Peroxide (H 2 O 2 ) in Water and in Organic Solvents Using Saccharomyces cerevisiae Meyen ex E.C. Hansen (Fungi: Ascomycota) | Semantic Scholar

SOLVED: You are given an aqueous solution of hydrogen peroxide that is 30.0% by mass and has a density of 1.11 g/mL What is the molality of the solution? Molar mass of

I have H2O2 of molecular wt 34.01gm and 30% w/v. What does it mean that I am not getting it and I want to prepare 0.1M solution, how can i? | ResearchGate